PADI DIVE THEORY
PADI dive theory – Decompression theory and the RDP is one of 5 subjects that will be tested on the PADI Instructor exam.
I have made these materials to help you to prepare for your PADI-Instructor exam or PADI Divemaster exam. If you choose to do your PADI Divemaster course or PADI IDC with Asia Scuba Instructors you will have access to many more materials in our ‘online classroom’.
The PADI Dive theory Studyguide and practice exams are also available in German language.
Physics
Light
The speed of light changes as it passes through different things such as air, glass and water. This affects the way we see things underwater with a diving mask.
As the light passes through the glass of the mask and the air space, the difference in speed causes the light rays to bend; this is called refraction. To the diver wearing a normal diving mask, objects appear to be larger and closer than they actually are – about 33% larger and closer by a ratio of 3:2.
Turbidity (Bad visibility underwater) can cause the diver to think that objects are further away than they actually are because they obscured by particles in the water. This phenomenon is known as visual reversal.

Light is also absorbed as it travels through water. The red colors are the first to disappear and blue last. That is why underwater pictures often look blue.
Sound
Sound travels four times faster in water than it does in air. This is because the water is a denser and more elastic medium than air – 800 times more dense. Because of this the diver’s brain perceives the sound as reaching both ears at the same time. This means divers cannot tell the direction the sound is coming from. The sound seems to come from everywhere at once or overhead.
Although divers cannot tell the direction, they can tell whether the sound is either closer or further away depending on its volume. Sound can travel very long distances underwater.
Heat
Water has a much higher heat capacity than air; this is its ability to draw heat away from another object such as a diver. Water conducts heat away from the diver twenty times faster than air for a given temperature. This is why a diver will chill quickly without an exposure suit even in warmer water.
The diver loses heat by three different methods while underwater:
Conduction has the most effect on the diver. This is caused by the water drawing heat by direct contact with the diver.
The second method is convection; this is caused by warm water rising (away from the diver) and being replaced by colder water. Like warm air rising to form clouds.
Radiation has the least effect on the diver; this is heat transmission without direct contact. Just like the rays from the sun.

Conduction

Convection

Radiation
Pressure
Pressure is measured in bar or atmospheres (atm), which are essentially the same.
Gauge pressure is the pressure of the water at a given depth (not counting the atmospheric pressure).
A pressure gauge on a scubatank shows gauge pressure. It will show zero when the tank is empty.
Absolute oder Ambient pressure is the water pressure plus the atmospheric pressure. (At sea level the atmospheric pressure is 1 bar/atm).
Pressure increases in sea water by 1 bar every 10 metres.
Pressure increases in fresh water by 1 bar every 10.3 metres.
To calculate gauge pressure in bar, simply multiply the depth of the water by 0.1 for seawater and by 0.097 for fresh water.
Examples:
To calculate the gauge pressure at 37m in sea water.
37 x 0.1 = 3.7 bar.
To calculate the gauge pressure at 16m in fresh water.
16 x 0.097 = 1.55 bar.
To calculate the absolute/ambient pressure in bar simply repeat the above procedure and then add 1 (providing you are calculating for sea level).
Examples:
To calculate the absolute pressure at 27m in sea water.
27 x 0.1 = 2.7
2.7 + 1 = 3.7 bar.
To calculate the absolute pressure at 22m in fresh water.
22 x 0.097 = 2.14
2.14 + 1 = 3.14 bar.
Remember that in all questions in physics of diving you will use absolute pressure in your calculations. Except if they just specifically ask you for the gauge pressure.

Pressure and volume
You cannot compress a liquid or a solid by applying greater pressure, but you can compress a gas as the molecules are further apart.
The volume of a gas is inversely proportional to the surrounding pressure on the gas.
If you take an inverted bucket down to 20m what would the volume of the air be inside it?
Pressure = 1 x 3
Volume = ⅓
If a balloon contains 15 litres of air at the surface; what would its volume be if taken down to 40m?
15 ÷ 5 = 3 litres.
If a balloon contains 7 litres of air at 30m and is then taken to the surface; what would its volume then be?
7 x 4 = 28 litres.
Remember; Ask yourself a question – ‘will the balloon get bigger or smaller ?’. Then you will know whether to multiply or divide.
If you move from one depth to another, it’s easiest to take it first to the surface and then back down to the new depth.
Example:
You take a balloon containing 5 litres of air from 35m up to 15m in salt water, what would its new volume be?
5 x 4.5 = 22.5 litres at the surface, then take it back down to 15m
22.5 ÷ 2.5 = 9 litres at 15m.
And the same problem in fresh water:
5 x 4.395 = 21.975 litres at the surface.
21.975 ÷ 2.455 = 8.95 litres at 15m
A balloon is sometimes called a flexible container. A scuba tank is referred to as an inflexible container – a scuba tank does not change volume or the amount of air it holds when changing depth.

Partial Pressures
In a mixture of gases, each gas exerts a pressure proportional to the percentage of that gas in the mixture. We call this partial pressure.
Air is a mixture of gases, containing, 21% Oxygen and 79% Nitrogen. At the surface the pressure is 1 bar. So the partial pressure of oxygen is 0.21 bar and the partial pressure of nitrogen is 0.79 bar.
As we go deeper the absolute pressure increases but the percentage of gasses remains the same.
To calculate the partial pressure of a gas at depth divide the percentage of the gas in the mix by 100 and multiply by the absolute pressure at that depth.
What is the partial pressure of oxygen in air at 30m?
21 ÷ 100 = 0.21
0.21 x 4 = 0.84 bar.
What is the partial pressure of nitrogen at 25m with EANx32?
(EANx32 has 32% Oxygen therefore 68% Nitrogen)
68 ÷ 100 = 0.68
0.68 x 3.5 = 2.38 bar.
Another type question related to partial pressures and contaminated air is this:
If a diver breathes air containing 0.5% carbon monoxide (CO) at 30m it would have the same effect as breathing______ % carbon monoxide at the surface. In this case you multiply the equation out :
0.5% x 4 bar = 2.0%
But if the question asks how many percent CO the diver breathes at 30m the answer would be 0.5% the same as the surface. Read the question!
Pressure and Absorption of Gases
If the pressure of a gas in contact with a liquid is increased the gas will dissolve into the liquid until a state of equilibrium is reached. (Saturation).
If the pressure of a gas in contact with a liquid is decreased the gas will come out of the liquid (supersaturation) if this happens quickly bubbles will form in the liquid.
This causes decompression sickness.
Sinking or floating
If an object is neutrally buoyant in salt water it will sink in fresh water.
If an object is neutrally buoyant in fresh water it will float in salt water.
If an object is positively or negatively buoyant in either fresh or salt water, you cannot determine exactly what will happen when placed from fresh to salt or vice versa unless you know exactly how much positive or negative buoyancy the object has.
Pressure, Temperature & Volume relationships
As a rule of thumb, in a scuba tank the pressure changes by 0.6 bar the for every degree Celsius change.
For flexible containers like balloons, the pressure remains the same and the volume changes.
A scuba tank is filled to 200 bar at 15 degrees Celsius, it is then left in the sun at a temperature of 30 degrees Celsius – what would the pressure in the tank now be?
30 – 15 = 15 degrees change
15 x 0.6 = 9 bar change upwards
New pressure = 209 bar.
A scuba tank is filled to 210 bar at a temperature of 35 degrees C and is then taken into water of 5 degrees C what would the pressure in the tank now be?
35 – 5 = 30
30 x 0.6 = 18
210 – 18 = 192 bar.
Buoyancy
When you push an object under water,
the water pushes back with a force that is equal to the weight of the water that is displaced.
Fresh water questions’ are relatively easy. Because 1 liter weighs 1 kilo, the calculation is very simple.
Kilos and liters is the same number.
If an object has a volume of 5 liters, the water pushes it up with 5 kilos.
In seawater it is also easy:
But 1 liter of seawater weighs 1.03 kilo.
Kilos and liters are not the same number.
5 liters in a liftbag gives it 5 x 1.03 = 5.15 kilos of lift.
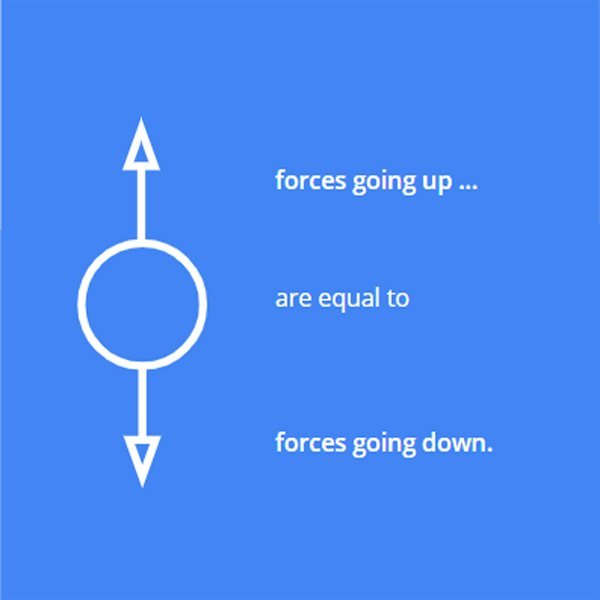
It can help to draw a little scheme to visualise this.
An anker weighs 220 kg. It displaces 100 liters of water. What is the minimum volume needed to lift this anker?
Fresh water:
220 – 100 = 120 liter
Seawater:
220 ÷ 1.03 – 100 = 113.6 liter
Example:
My camera and housing weighs 4.4 kg. How much weight do I need, to make it neutrally buoyant if the housing displaces 5.2 liter?
Fresh water:
5.2 – 4.4 = 0.8 kg
Seawater:
5.2 x 1.03 – 4.4 =
5.4 – 4.4 = 1 kg
At one point, we have to convert liters to kilos or vice versa.
To make life a bit easier, look at what the question is asking for.
Example:
A diver in full scuba gear weighs 85 kg. He displaces 90 liters of seawater. How many weights does he need to carry to become neutrally buoyant?
Convert to kg:
Force down:
85 kg + ??
Force up:
90 x 1.03 kg = 92.7 kg
85 kg + ?? = 92.7 kg
92.7 kg – 85 kg = 7.7 kg
Example:
A diver in full scuba gear weighs 95 kg. He displaces 90 liters of seawater. How much air does he need in his BCD to become neutrally buoyant?
Convert to liters:
Force down
95 ÷ 1.03 = 92.2 liters (92.2 liters of seawater weighs 95 kg !)
Force up:
90 liters + ??
90 liters + ?? = 92.2 liters
?? = 2.2 liters
In words:
The diver wants to become neutrally buoyant. He weighs 95 kg so he needs to be lifted up with 95 kg force. For this he needs to displace 92.2 liters of seawater with his BCD. He already displaces 90 liters of seawater with his body and equipment so he needs 2.2 liters more.
Buoyancy and pressure combined
Some questions say that an object is at a specific depth.
Does the depth matter? No.
If an object weighs 10 kilos at 12 meters, it weighs 10 kilos at 45 meters.
You still need the same volume to lift it.
However, the volume in your liftbag will increase when the object is taken to the surface (because the pressure is less).
When asked “What is the volume of the liftbag, upon reaching the surface” or “How much air from the surface needs to be pumped in the liftbag” then depth does matter.
You use a liftbag to lift an object that weighs 10 kg and displaces 2 liters of seawater.
The object lies at 30 meter depth.
What will be the air volume in the liftbag upon reaching the surface?
To lift 10 kg we need to displace
10 ÷ 1.03 = 9.7 liter
The object displaces 2 liter, so we need 7.7 liters more.
At 30 meters the pressure is 4 Bar so the volume on the surface will be:
4 x 7.7 liters = 30.8 liter

